Physics | Chemistry | Maths
460+ Hrs. of Extensive Studies for Physics, Chemistry and Maths with aprox. 150+ Hrs. devoted on each subject. Along with this Class Tests, Assignments, Doubt Sessions, Syllabus Revision in the end also included. Our expert faculties are Masters Qualified in their specialized subject and having more than 10+ years of experience and expertise to provide in depth knowledge for the subject concerned.
Senior Secondary stage of school education is a stage of transition from general education to discipline-based focus on curriculum. The present updated syllabus keeps in view the rigor and depth of disciplinary approach as well as the comprehension level of learners. Due care has also been taken that the syllabus is comparable to the international standards.
Pattern of Studies
● Interactive Classes
● Home Assignments
● Class Notes
● Periodic Class Tests
● Student's Monthly Report
● One to One Attention
● Doubt Classes
● Syllabus Revision
Report Analysis Parameters
● Tests Marks
● Attendance
● Class Discipline
● Class Response
● Home Assignments
● Student's Comparison with Class
● Last 3 Months Performance
● Special Remarks
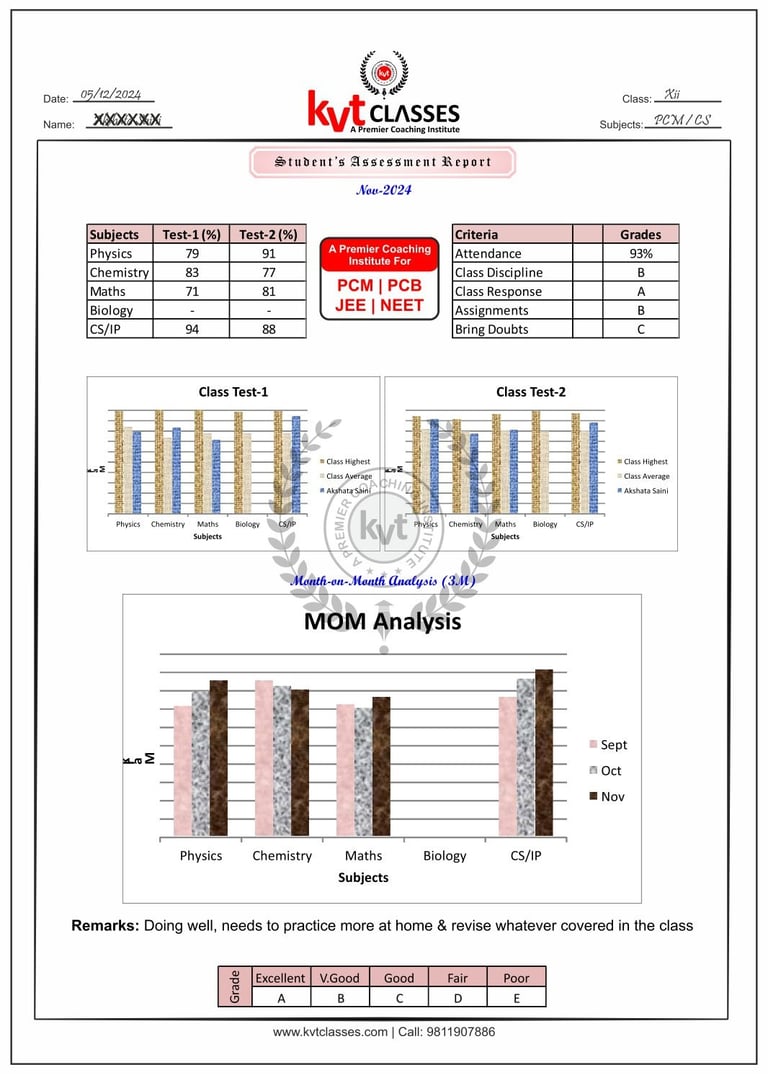
Student's Sample Report
Class Tests
● Two tests per subject in a month
● Test schedule on Saturday in extra hours
● Result within 1 week of test conduct
● Test discussion on result day
● Mistakes rectification briefing

Class-XI
Trusted by Many Prestigious School Students
Students from many prestigious Schools in the vicinity trusted KVT Classes for their Education needs because we believe in giving Quality Education. We get students all across South Delhi who study in different Schools mentioned below..


Star Performers KVT
Students who made us proud


Conduct of Classes
● On board explanation with notes
● Make Concept clear with practical examples
● Interactive class sessions
● Home work / Assignment at the end of the class
● Home work / Assignment check in the next class
● Quick recap of previous class in the next class
● Two tests per month/subject on Saturdays
● Class duration 1 Hr/sub alternate days (3Hrs./wk)
● Extra classes provided when needed
Classes Schedule 2024-25
Science Students Class XI-XII
Note: Class Tests will be conducted on Saturday in extra hours
Syllabus & Marks Weightage [ Physics]
Syllabus & Marks Weightage [ Chemistry ]
Syllabus & Marks Weightage [ Maths ]
Detailed Syllabus [ Physics ] XI
Unit I: Physical World and Measurement 08 Periods
Chapter–2: Units and Measurements
Need for measurement: Units of measurement; systems of units; SI units, fundamental and derived units. significant figures. Dimensions of physical quantities, dimensional analysis and its applications.
Unit II: Kinematics 24 Periods
Chapter–3: Motion in a Straight Line
Frame of reference, Motion in a straight line, Elementary concepts of differentiation and integration for describing motion, uniform and nonuniform motion, and instantaneous velocity, uniformly accelerated motion, velocity - time and position-time graphs. Relations for uniformly accelerated motion (graphical treatment).
Chapter–4: Motion in a Plane
Scalar and vector quantities; position and displacement vectors, general vectors and their notations; equality of vectors, multiplication of vectors by a real number; addition and subtraction of vectors, Unit vector; resolution of a vector in a plane, rectangular components, Scalar and Vector product of vectors. Motion in a plane, cases of uniform velocity and uniform acceleration projectile motion, uniform circular motion.
Unit III: Laws of Motion 14 Periods
Chapter–5: Laws of Motion
Intuitive concept of force, Inertia, Newton's first law of motion; momentum and Newton's second law of motion; impulse; Newton's third law of motion. Law of conservation of linear momentum and its applications. Equilibrium of concurrent forces, Static and kinetic friction, laws of friction, rolling friction, lubrication. Dynamics of uniform circular motion: Centripetal force, examples of circular motion (vehicle on a level circular road, vehicle on a banked road).
Unit IV: Work, Energy and Power 14 Periods
Chapter–6: Work, Energy and Power
Work done by a constant force and a variable force; kinetic energy, work energy theorem, power. Notion of potential energy, potential energy of a spring, conservative forces: non-conservative forces, motion in a vertical circle; elastic and inelastic collisions in one and two dimensions.
Unit V: Motion of System of Particles and Rigid Body 18 Periods
Chapter–7: System of Particles and Rotational Motion
Centre of mass of a two-particle system, momentum conservation and Centre of mass motion. Centre of mass of a rigid body; centre of mass of a uniform rod. Moment of a force, torque, angular momentum, law of conservation of angular momentum and its applications. Equilibrium of rigid bodies, rigid body rotation and equations of rotational motion, comparison of linear and rotational motions. Moment of inertia, radius of gyration, values of moments of inertia for simple geometrical objects (no derivation).
Unit VI: Gravitation 12 Periods
Chapter–8: Gravitation
Kepler's laws of planetary motion, universal law of gravitation. Acceleration due to gravity and its variation with altitude and depth. Gravitational potential energy and gravitational potential, escape speed, orbital velocity of a satellite.
Unit VII: Properties of Bulk Matter 24 Periods
Chapter–9: Mechanical Properties of Solids
Elasticity, Stress-strain relationship, Hooke's law, Young’s modulus, bulk modulus, shear modulus of rigidity (qualitative idea only), Poisson's ratio; elastic energy.
Chapter–10: Mechanical Properties of Fluids
Pressure due to a fluid column; Pascal's law and its applications (hydraulic lift and hydraulic brakes), effect of gravity on fluid pressure. Viscosity, Stokes' law, terminal velocity, streamline and turbulent flow, critical velocity, Bernoulli's theorem and its simple applications. Surface energy and surface tension, angle of contact, excess of pressure across a curved surface, application of surface tension ideas to drops, bubbles and capillary rise.
Chapter–11: Thermal Properties of Matter
Heat, temperature, thermal expansion; thermal expansion of solids, liquids and gases, anomalous expansion of water; specific heat capacity; Cp, Cv - calorimetry; change of state - latent heat capacity. Heat transfer-conduction, convection and radiation, thermal conductivity, qualitative ideas of Blackbody radiation, Wein's displacement Law, Stefan's law .
Unit VIII: Thermodynamics 12 Periods
Chapter–12: Thermodynamics
Thermal equilibrium and definition of temperature, zeroth law of thermodynamics, heat, work and internal energy. First law of thermodynamics, Second law of thermodynamics: gaseous state of matter, change of condition of gaseous state -isothermal, adiabatic, reversible, irreversible, and cyclic processes.
Unit IX: Behavior of Perfect Gases and Kinetic Theory of Gases 08 Periods
Chapter–13: Kinetic Theory
Equation of state of a perfect gas, work done in compressing a gas. Kinetic theory of gases - assumptions, concept of pressure. Kinetic interpretation of temperature; rms speed of gas molecules; degrees of freedom, law of equi-partition of energy (statement only) and application to specific heat capacities of gases; concept of mean free path, Avogadro's number.
Unit X: Oscillations and Waves 26 Periods
Chapter–14: Oscillations
Periodic motion - time period, frequency, displacement as a function of time, periodic functions and their applications. Simple harmonic motion (S.H.M) and its equations of motion; phase; oscillations of a loaded spring- restoring force and force constant; energy in S.H.M. Kinetic and potential energies; simple pendulum derivation of expression for its time period.
Chapter–15: Waves
Wave motion: Transverse and longitudinal waves, speed of travelling wave, displacement relation for a progressive wave, principle of superposition of waves, reflection of waves, standing waves in strings and organ pipes, fundamental mode and harmonics, Beats.
Detailed Syllabus [ Chemistry ] XI
Unit I: Some Basic Concepts of Chemistry 12 Periods
General Introduction: Importance and scope of Chemistry.
Nature of matter, laws of chemical combination, Dalton's atomic theory: concept of elements, atoms and molecules. Atomic and molecular masses, mole concept and molar mass, percentage composition, empirical and molecular formula, chemical reactions, stoichiometry and calculations based on stoichiometry.
Unit II: Structure of Atom 14 Periods
Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson's model and its limitations. Rutherford's model and its limitations, Bohr's model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie's relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p and d orbitals, rules for filling electrons in orbitals - Aufbau principle, Pauli's exclusion principle and Hund's rule, electronic configuration of atoms, stability of half-filled and completely filled orbitals.
Unit III: Classification of Elements and Periodicity in Properties 08 Periods
Significance of classification, brief history of the development of periodic table, modern periodic law and the present form of periodic table, periodic trends in properties of elements -atomic radii, ionic radii, inert gas radii, Ionization enthalpy, electron gain enthalpy, electronegativity, valency. Nomenclature of elements with atomic number greater than 100.
Unit IV: Chemical Bonding and Molecular Structure 14 Periods
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization, involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules(qualitative idea only), Hydrogen bond.
Unit V: Chemical Thermodynamics 16 Periods
Concepts of System and types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions.
First law of thermodynamics -internal energy and enthalpy, heat capacity and specific heat, measurement of U and H, Hess's law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. Second law of Thermodynamics (brief introduction)
Introduction of entropy as a state function, Gibb's energy change for spontaneous and non- spontaneous processes, criteria for equilibrium. Third law of thermodynamics (brief introduction).
Unit VI: Equilibrium 14 Periods
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium - Le Chatelier's principle, ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of poly basic acids, acid strength, concept of pH, hydrolysis of salts (elementary idea), buffer solution, Henderson Equation, solubility product, common ion effect (with illustrative examples).
Unit VII: Redox Reactions 06 Periods
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, in terms of loss and gain of electrons and change in oxidation number, applications of redox reactions.
Unit VIII: Organic Chemistry -Some Basic Principles and Techniques 14 Periods
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions.
Unit IX: Hydrocarbons 12 Periods
Classification of Hydrocarbons
Aliphatic Hydrocarbons:
Alkanes - Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis.
Alkenes - Nomenclature, structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov's addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition.
Alkynes - Nomenclature, structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of - hydrogen, halogens, hydrogen halides and water.
Aromatic Hydrocarbons:
Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft's alkylation and acylation, directive influence of functional group in monosubstituted benzene. Carcinogenicity and toxicity.
Detailed Syllabus [ Maths ] XI
Unit-I: Sets and Functions
1.Sets(20)Periods
Sets and their representations, Empty set, Finite and Infinite sets, Equal sets, Subsets, Subsets of a set of real numbers especially intervals (with notations). Universal set. Venn diagrams. Union and Intersection of sets. Difference of sets. Complement of a set. Properties of Complement.
2.Relations & Functions(20)Periods
Ordered pairs. Cartesian product of sets. Number of elements in the Cartesian product of two finite sets. Cartesian product of the set of reals with itself (upto R x R x R).Definition of relation, pictorial diagrams, domain, co-domain and range of a relation. Function as a special type of relation. Pictorial representation of a function, domain, co-domain and range of a function. Real valued functions, domain and range of these functions, constant, identity, polynomial, rational, modulus, signum, exponential, logarithmic and greatest integer functions, with their graphs. Sum, difference, product and quotients of functions.
3.Trigonometric Functions(20)Periods
Positive and negative angles. Measuring angles in radians and in degrees and conversion from one measure to another. Definition of trigonometric functions with the help of unit circle. Truth of the identity sin2x + cos2x = 1, for all x. Signs of trigonometric functions. Domain and range of trigonometric functions and their graphs. Expressing sin (x±y) and cos (x±y) in terms of sinx, siny, cosx & cosy and their simple applications. Deducing identities like the following: tan(x±y)=tanx±tany1∓tanxtany ,cot(x±y)=cotxcoty ∓1coty±cotxsinα±sinβ=2sin12(α±β)cos12(α∓β) cosα+cosβ=2cos12(α+β)cos12(α−β) 𝑐𝑜𝑠𝛼−𝑐𝑜𝑠𝛽=−2𝑠𝑖𝑛12(𝛼+𝛽)𝑠𝑖𝑛12(𝛼−𝛽) Identities related to sin2x, cos2x, tan2 x, sin3x, cos3x and tan3x.
Unit-II: Algebra
1.Complex Numbers and Quadratic Equations(10)Periods
Need for complex numbers, especially√−1, to be motivated by inability to solve some of the quadratic equations. Algebraic properties of complex numbers. Argand plane
2.Linear Inequalities(10)Periods
Linear inequalities. Algebraic solutions of linear inequalities in one variable and their representation on the number line.
3.Permutations and Combinations(10)Periods
Fundamental principle of counting. Factorial n. (n!) Permutations and combinations, derivation of Formulae for nPr and nCr and their connections, simple applications.
4.Binomial Theorem(10)Periods
Historical perspective, statement and proof of the binomial theorem for positive integral indices. Pascal’s triangle, simple applications.
5.Sequence and Series(10)Periods
Sequence and Series. Arithmetic Mean (A.M.) Geometric Progression (G.P.), general term of a G.P., sum of n terms of a G.P., infinite G.P. and its sum, geometric mean (G.M.), relation between A.M. and G.M.
Unit-III: Coordinate Geometry
1.Straight Lines(15)Periods
Brief recall of two dimensional geometry from earlier classes. Slope of a line and angle between two lines. Various forms of equations of a line: parallel to axis, point -slope form, slope-intercept form, two-point form, intercept form, Distance of a point from a line.
2.Conic Sections(25)Periods
Sections of a cone: circles, ellipse, parabola, hyperbola, a point, a straight line and a pair of intersecting lines as a degenerated case of a conic section. Standard equations and simple properties of parabola, ellipse and hyperbola. Standard equation of a circle.
3.Introduction to Three-dimensional Geometry(10)Periods
Coordinate axes and coordinate planes in three dimensions. Coordinates of a point. Distance between two points.
Unit-IV: Calculus
1.Limits and Derivatives(40)Periods
Derivative introduced as rate of change both as that of distance function and geometrically. Intuitive idea of limit. Limits of polynomials and rational functions trigonometric, exponential and logarithmic functions. Definition of derivative relate it to scope of tangent of the curve, derivative of sum, difference, product and quotient of functions. Derivatives of polynomial and trigonometric functions.
Unit-V Statistics and Probability
1.Statistics(20)Periods
Measures of Dispersion: Range, Mean deviation, variance and standard deviation of ungrouped/grouped data.
2.Probability(20)Periods
Events; occurrence of events, ‘not’, ‘and’ and ‘or’ events, exhaustive events, mutually exclusive events, Axiomatic (set theoretic) probability, connections with other theories of earlier classes. Probability of an event, probability of ‘not’, ‘and’ and ‘or’ events.
